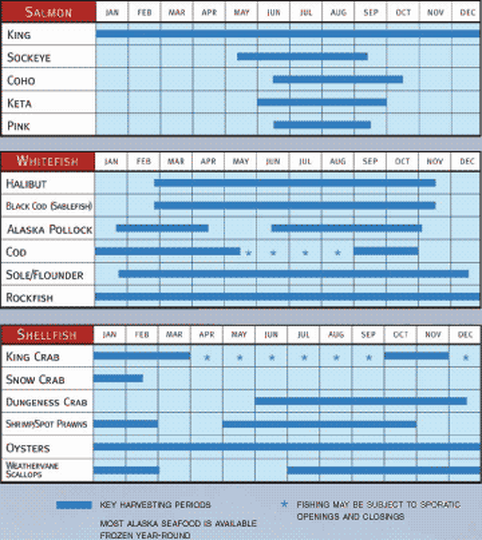
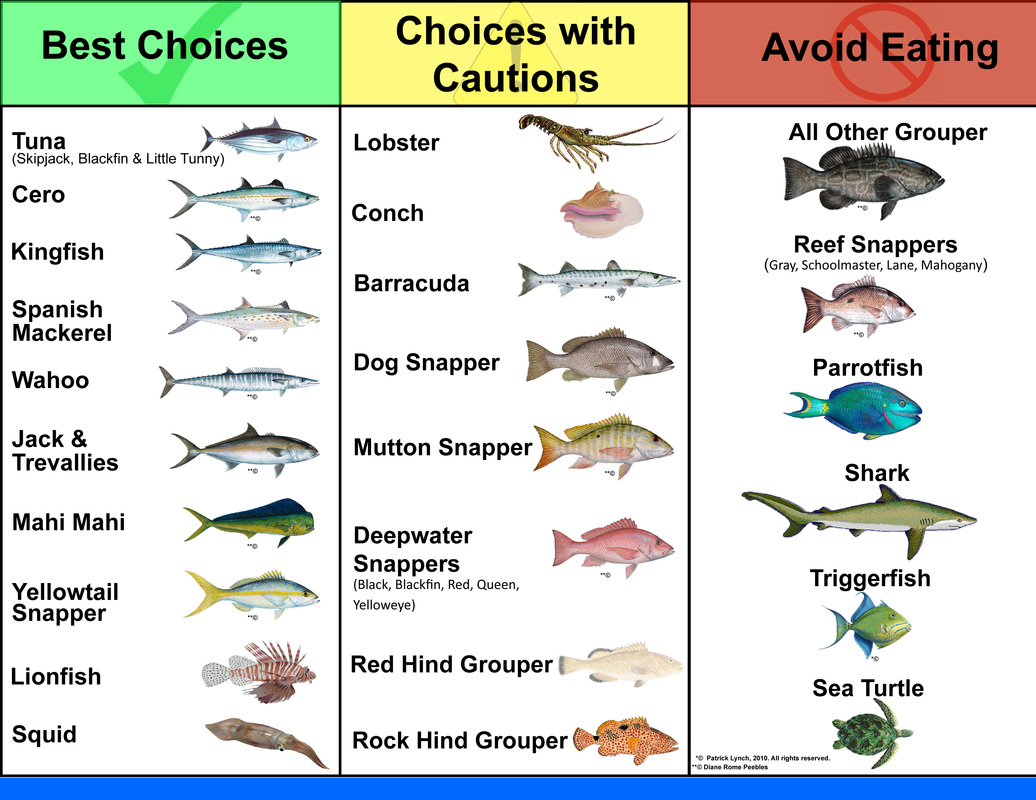
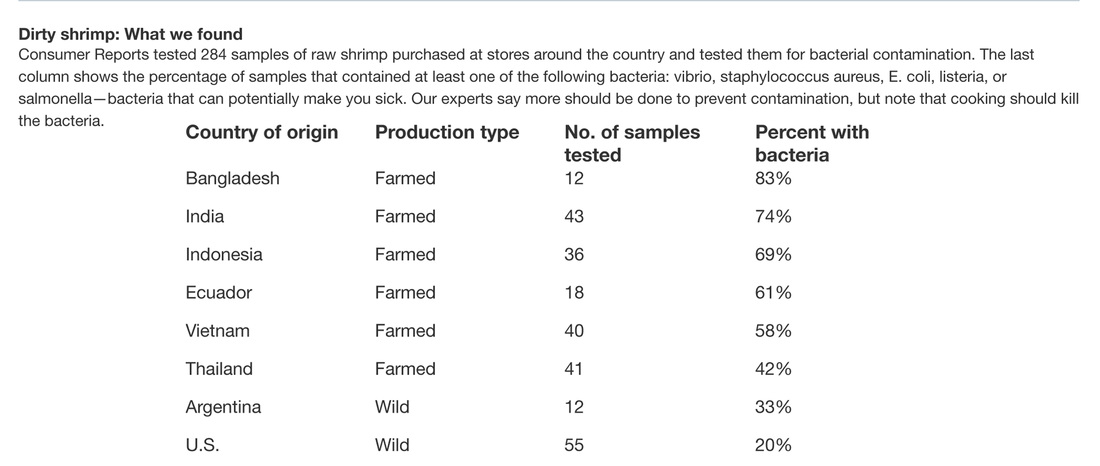
|
|
|
|
WORRY ABOUT AND QUESTION WHERE YOUR SHRIMP COMES FROM. IT MAY NOT BE PRETTY!
The USA does not test imported shrimp enough to catch contamination. See article below that points out once Japan started testing all shrimp imports it found way too much shrimp with high antibiotic levels to continue so shifted supplier to India instead. With the Trans Pacific Partnership, rules will get more lax as we allow for more imports. Click to learn more.
Watch this video to see the conditions shrimp are raised in Vietnam.
Japan may stop Vietnamese shrimp imports due to high levels of antibiotics.

HA NOI (VNS) – Japanese businesses are planning to import shrimp from India and Indonesia instead of Viet Nam, due to the excessively high levels of oxytetracycline (OTC) in Vietnamese shrimp.
According to the Viet Nam Association of Seafood Exporters and Producers (VASEP), the decision was made due to the excessively high levels of OTC that were continuing to be detected in Vietnamese shrimp shipments, despite prior warnings and the public knowledge that virtually all Vietnamese shrimp exports were being tested for the antibiotic.
VASEP asked the Ministry of Agriculture and Rural Development's Directorate of Fisheries to strictly control shrimp cultivating areas to prevent such a situation.
VASEP's figure showed that 11 shrimp shipments to the EU and Japan were returned in the first four months of the year because of high OTC levels.
It said the number of shrimp shipments being returned by Japan had risen since it started testing for OTC in all Vietnamese shrimps.
The association also warned that Japanese importers are planning to import shrimp from the two above-mentioned countries as they have taken measures to reduce the OTC level.
Japanese importers have also guided shrimp processing factories in India to process shrimps to switch the orders from Viet Nam.
Two weeks ago, VASEP said the EU discovered OTC levels in some shrimp shipments from Viet Nam to be higher than the allowed level of 0.1ppm.
It warned that the EU would consider applying stricter measures on Vietnamese shrimp if Viet Nam did not improve the situation.
Earlier this year, the association forecast that shrimp exports could reach US$3 billion this year if the issues of breed and presence of chemicals were paid due attention.
It also warned that unless the local shrimp businesses strengthened self-regulation of OTC, they would fail to penetrate the Japanese market as well as to meet the target of making Viet Nam one of the top three shrimp exporters in the world. -- VNS
Read more at http://vietnamnews.vn/economy/254296/japan-may-stop-vietnamese-shrimp-imports.html#0q1MyCj0ZvY4uTEy.99
According to the Viet Nam Association of Seafood Exporters and Producers (VASEP), the decision was made due to the excessively high levels of OTC that were continuing to be detected in Vietnamese shrimp shipments, despite prior warnings and the public knowledge that virtually all Vietnamese shrimp exports were being tested for the antibiotic.
VASEP asked the Ministry of Agriculture and Rural Development's Directorate of Fisheries to strictly control shrimp cultivating areas to prevent such a situation.
VASEP's figure showed that 11 shrimp shipments to the EU and Japan were returned in the first four months of the year because of high OTC levels.
It said the number of shrimp shipments being returned by Japan had risen since it started testing for OTC in all Vietnamese shrimps.
The association also warned that Japanese importers are planning to import shrimp from the two above-mentioned countries as they have taken measures to reduce the OTC level.
Japanese importers have also guided shrimp processing factories in India to process shrimps to switch the orders from Viet Nam.
Two weeks ago, VASEP said the EU discovered OTC levels in some shrimp shipments from Viet Nam to be higher than the allowed level of 0.1ppm.
It warned that the EU would consider applying stricter measures on Vietnamese shrimp if Viet Nam did not improve the situation.
Earlier this year, the association forecast that shrimp exports could reach US$3 billion this year if the issues of breed and presence of chemicals were paid due attention.
It also warned that unless the local shrimp businesses strengthened self-regulation of OTC, they would fail to penetrate the Japanese market as well as to meet the target of making Viet Nam one of the top three shrimp exporters in the world. -- VNS
Read more at http://vietnamnews.vn/economy/254296/japan-may-stop-vietnamese-shrimp-imports.html#0q1MyCj0ZvY4uTEy.99
Reason for Alert:
There has been extensive commercialization and increased consumption of aquaculture seafood products worldwide. Aquacultured seafood has become the fastest growing sector of the world food economy, accounting for approximately half of all seafood production worldwide. Approximately 80% of the seafood consumed in the U.S. is imported from approximately 62 countries. Over 40% of that seafood comes from aquaculture operations. As the aquaculture industry continues to grow and compete with wild-caught seafood products, concerns regarding the use of unapproved animal drugs and unsafe chemicals and the misuse of animal drugs in aquaculture operations have increased substantially. China is the largest producer of aquacultured seafood in the world, accounting for 70% of the total production and 55% of the total value of aquacultured seafood exported around the world. China is currently the third largest exporter of seafood to the U.S. Shrimp represent the top ten most consumed seafood products in the U.S. The use of unapproved antibiotics or chemicals in aquaculture raises significant public health concerns. There is clear scientific evidence that the use of antibiotics or chemicals, such as malachite green, nitrofurans, fluoroquinolones, and gentian violet during the various stages of aquaculture can result in the presence of residues of the parent compound or its metabolites in the edible portion of the aquacultured seafood. The presence of antibiotic residues may contribute to an increase of antimicrobial resistance in human pathogens. Moreover, prolonged exposure to nitrofurans, malachite green, and gentian violet has been shown to have a carcinogenic affect. In the United States, use of malachite green, nitrofurans, fluoroquinolones, or gentian violet as drugs in food-producing animals would require an approved new animal drug application under Section 512 of the Federal Food, Drug, and Cosmetic Act (FFDCA). FDA has not approved these antibiotics for use as drugs in aquacultured animals. Therefore, if they are used in aquaculture with an intent that they treat disease in, or affect the structure or function of, any aquacultured animal, they are considered to be unsafe new animal drugs within the meaning of Section 512, and the presence of their residues in seafood adulterates the seafood under 402(a)(2)(C)(ii) of the FFDCA. Furthermore, malachite green, nitrofurans, fluoroquinolones, and gentian violet are not generally recognized as safe under any conditions of intended use that may reasonably be expected to result in their becoming a component of food. Therefore, if intended for any such use, they are unsafe food additives within the meaning of section 409 of the FDCA and would render the food adulterated under section 402(a)(2)(C)(i). FDA has several existing Import alerts related to unapproved drugs in seafood dating back to November of 2001 (IA #16-124 DWPE of Seafood Products Due to Unapproved Drugs, IA #16-129 DWPE of Seafood Products Due to Nitrofurans, and IA #16-130 DWPE of Eel from China Due to the presence of Malachite Green). Based on an increased monitoring of imported aquacultured seafood from October 1, 2006, through May 31, 2007, FDA continued to find residues of unapproved new animal drugs and/or unsafe food additives in seafood imported from China. During that period, FDA tested 89 samples consisting of catfish, Basa, shrimp, dace and eel from China. Twenty two (22) of the 89 samples (25%) were found to contain drug residues. These residues include nitrofurans detected in shrimp at levels above 1 ppb; malachite green detected in dace, eel and catfish/Basa fish at levels ranging from 2.1 to 122 ppb; gentian violet detected in eel and catfish at levels ranging from 2.5 ppb to 26.9 ppb and fluoroquinolones in catfish/Basa at level ranging from 1.9 to 6.5 ppb. Furthermore, Chinese authorities have acknowledged permitting the use of fluoroquinolones in aquaculture. Although the use of some animal drugs (nitrofurans and malachite green)in aquaculture has been prohibited by Chinese authorities since 2002, FDA continues to find residues of these and other animal drugs in shipments of aquacultured seafood products from China.
There has been extensive commercialization and increased consumption of aquaculture seafood products worldwide. Aquacultured seafood has become the fastest growing sector of the world food economy, accounting for approximately half of all seafood production worldwide. Approximately 80% of the seafood consumed in the U.S. is imported from approximately 62 countries. Over 40% of that seafood comes from aquaculture operations. As the aquaculture industry continues to grow and compete with wild-caught seafood products, concerns regarding the use of unapproved animal drugs and unsafe chemicals and the misuse of animal drugs in aquaculture operations have increased substantially. China is the largest producer of aquacultured seafood in the world, accounting for 70% of the total production and 55% of the total value of aquacultured seafood exported around the world. China is currently the third largest exporter of seafood to the U.S. Shrimp represent the top ten most consumed seafood products in the U.S. The use of unapproved antibiotics or chemicals in aquaculture raises significant public health concerns. There is clear scientific evidence that the use of antibiotics or chemicals, such as malachite green, nitrofurans, fluoroquinolones, and gentian violet during the various stages of aquaculture can result in the presence of residues of the parent compound or its metabolites in the edible portion of the aquacultured seafood. The presence of antibiotic residues may contribute to an increase of antimicrobial resistance in human pathogens. Moreover, prolonged exposure to nitrofurans, malachite green, and gentian violet has been shown to have a carcinogenic affect. In the United States, use of malachite green, nitrofurans, fluoroquinolones, or gentian violet as drugs in food-producing animals would require an approved new animal drug application under Section 512 of the Federal Food, Drug, and Cosmetic Act (FFDCA). FDA has not approved these antibiotics for use as drugs in aquacultured animals. Therefore, if they are used in aquaculture with an intent that they treat disease in, or affect the structure or function of, any aquacultured animal, they are considered to be unsafe new animal drugs within the meaning of Section 512, and the presence of their residues in seafood adulterates the seafood under 402(a)(2)(C)(ii) of the FFDCA. Furthermore, malachite green, nitrofurans, fluoroquinolones, and gentian violet are not generally recognized as safe under any conditions of intended use that may reasonably be expected to result in their becoming a component of food. Therefore, if intended for any such use, they are unsafe food additives within the meaning of section 409 of the FDCA and would render the food adulterated under section 402(a)(2)(C)(i). FDA has several existing Import alerts related to unapproved drugs in seafood dating back to November of 2001 (IA #16-124 DWPE of Seafood Products Due to Unapproved Drugs, IA #16-129 DWPE of Seafood Products Due to Nitrofurans, and IA #16-130 DWPE of Eel from China Due to the presence of Malachite Green). Based on an increased monitoring of imported aquacultured seafood from October 1, 2006, through May 31, 2007, FDA continued to find residues of unapproved new animal drugs and/or unsafe food additives in seafood imported from China. During that period, FDA tested 89 samples consisting of catfish, Basa, shrimp, dace and eel from China. Twenty two (22) of the 89 samples (25%) were found to contain drug residues. These residues include nitrofurans detected in shrimp at levels above 1 ppb; malachite green detected in dace, eel and catfish/Basa fish at levels ranging from 2.1 to 122 ppb; gentian violet detected in eel and catfish at levels ranging from 2.5 ppb to 26.9 ppb and fluoroquinolones in catfish/Basa at level ranging from 1.9 to 6.5 ppb. Furthermore, Chinese authorities have acknowledged permitting the use of fluoroquinolones in aquaculture. Although the use of some animal drugs (nitrofurans and malachite green)in aquaculture has been prohibited by Chinese authorities since 2002, FDA continues to find residues of these and other animal drugs in shipments of aquacultured seafood products from China.
|
|