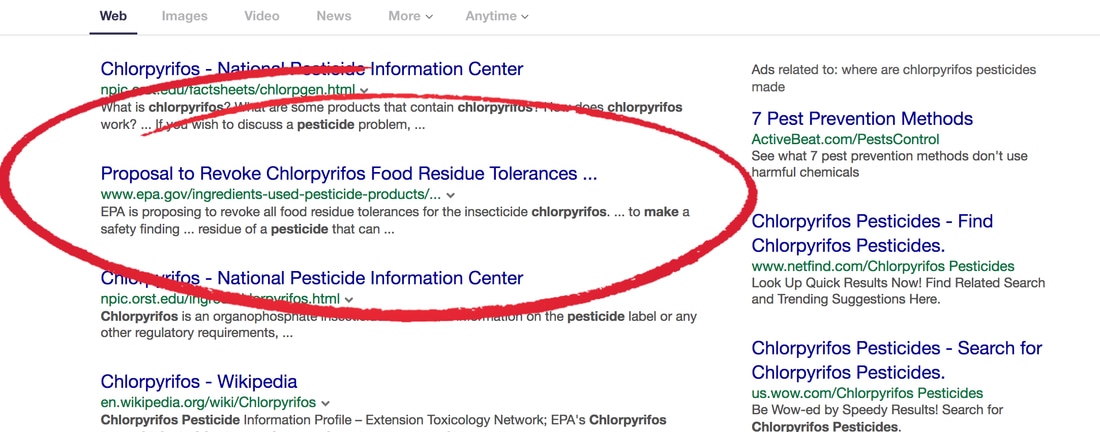
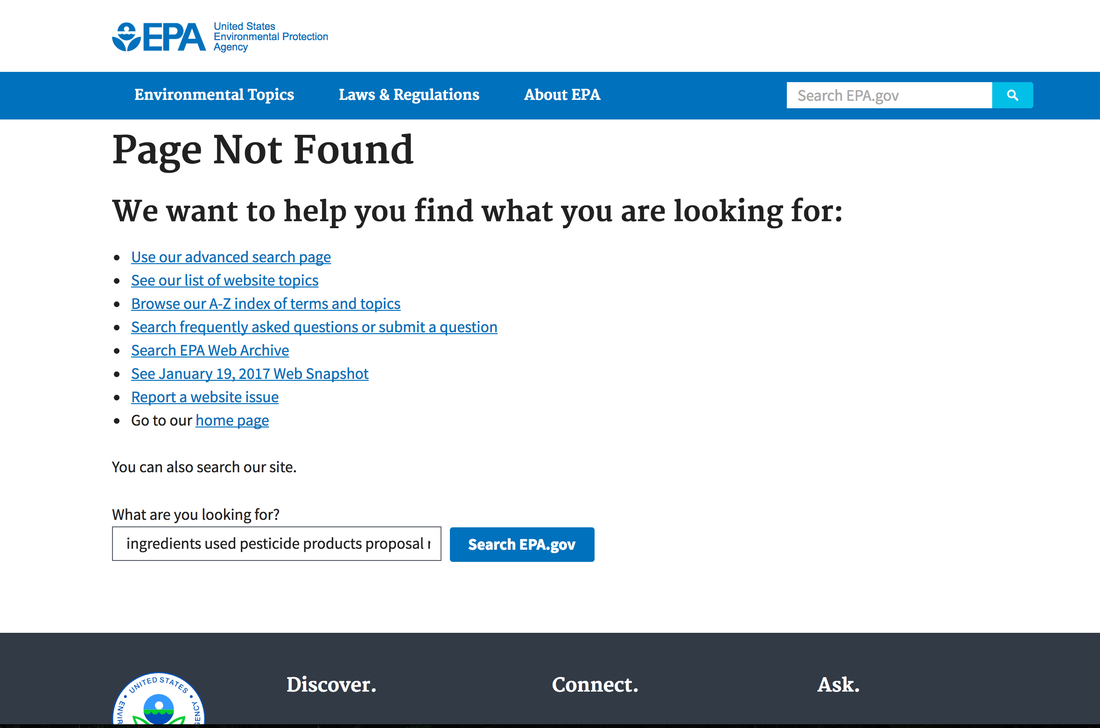
EPA has been ordered to take down information about the hazards of Chlorpyrifos, a highly toxic pesticide that causes birth defects in our children, robs them of a higher IQ, and causes slow development and autism. People are easier to control if you destroy their brain development and less likely to rebel. Read about information previously posted on the EPA website that was asking for comments. Everything was in place to phase out it's use until it was reversed by Trump. Mother Jones Reports |
AuthorElaine McFadden, MPH, RD, is the host for "Smart Health Talk Radio Show, Thursdays, 4:00-5:00, on KCAA Radio 1050 AM in Southern California. Elaine is a dietitian dedicated to bringing consumers information that can improve their life and save them money. Archives
March 2019
Categories
All
|





 RSS Feed
RSS Feed
